
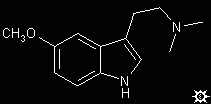
#38. 5-MEO-DMT
TRYPTAMINE, 5-METHOXY-N,N-DIMETHYL; INDOLE, 5-METHOXY-3-[2-(DIMETHYLAMINO)ETHYL]; 5-METHOXY-N,N-DIMETHYLTRYPTAMINE; 5-METHOXY-3-[2-(DIMETHYLAMINO)ETHYL]INDOLE; N,N,O-TRIMETHYLSEROTONIN; N,N,O-TMS; BUFOTENINE METHYL ETHER; O-METHYLBUFOTENINE; OMB
|
| [3D .jpg Image] |
To a well-stirred suspension of 11.7 g LAH in 350 mL anhydrous Et2O there was added, in small portions, a suspension of 18.5 g 5-methoxy-N,N-dimethylindol-3-ylglyoxylamide in 200 mL hot benzene. The last of the solids were rinsed in with anhydrous Et2O and the mixture held at reflux for 1.5 h. After cooling with an external ice bath, the reaction complex and excess hydride were decomposed by the cautious addition of H2O. The inorganic solids were removed by filtration, the filter cake washed with additional Et2O, the filtrate and washing were combined and dried over anhydrous MgSO4, and the solvents removed under vacuum. The residue was distilled at the KugelRohr to provide a colorless fraction distilling at 160-170 °C at 0.6 mm/Hg, that crystallized on cooling. There was thus obtained 12.8 g (78%) 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) which on recrystallization from hexane had a mp 69-70 °C. The hydrochloride salt can be made by passing a stream of hydrogen chloride gas through an Et2O solution of the base. It, upon recrystallization from EtOH / Et2O, had a mp of 145-146 °C.
DOSAGE : 6 - 20 mg, smoked; 2 - 3 mg, i.v.
DURATION : 1 - 2 hrs
QUALITATIVE COMMENTS : (with 6 mg, smoked) "I felt it in a minute -- not really light head, but the head feels close to the lower parts of the body -- close to the ground -- knees weak -- distinct shakes. I peaked at 2 or three minutes. It was quite intense, but not the max of DMT at 30 milligrams and no sensory close-out. Slight nausea on the drop-off -- I am glad I had not eaten anything. Overall comparison to DMT, more potent, slightly faster, but like DMT is largely a simple, stoning drug with no sensory contribution, no intellectual contribution. It's greatest contribution might be to provide a subject the vocabulary of an altered state of consciousness so that, with interesting and constructive drugs, these effects will be familiar, and thus not distractions."
(with 8 mg, smoked) "I was blown away, far away I might add, but only for 10 minutes and effects were gone by half an hour. During this episode mental activity was almost absent. I can't say I wasn't 'impressed' in some way, though it wasn't exactly what I expected. I had read reports with statements varying from 'dwarfs + elves' to 'conk on the head,' the latter of which relates more closely to my experience."
(with perhaps 10 mg, smoked) "Onset was gentle, perhaps over 15 minutes. I felt like all of my blood had turned to concrete. There were no noticeable visual effects, but my hearing was slightly diminished. The whole experience was over after 1 hour."
(with perhaps 15 mg, smoked) "I took a hit from the pipe with five-methoxy in it, and after the 8 to 10 seconds it took to carry the chemical to my brain I remember starting to fall over from my sitting position. My normal physical perceptions dissolved away from my awareness. My ears started to ring and I started to float off. I was acutely aware of a certain resonation of my aural perception, an electrical buzzing that fluctuated in synch with my visual perception. What I saw can only be described as a fantastically subtle multicolored phosphene, completely filling every area visually available. I say it in this way because I was simultaneously losing contact with my body, I could not tell if my eyes were open or shut, although I initially had the feeling that they were darting back and forth, from side to side. These feelings and sensations built up in intensity very quickly, a matter of seconds: I can remember this feeling of building intensity up to a point, and then I was not there in my body or in time. In the 10 to 15 minutes that my body was under the influence of the drug my mind was completely referenceless, there was no way for my consciousness to limit or gauge the stimuli my being was barraged with. I remember switching to a perception where the endless and intricate phosphene was love and the energy of light. I called upon those forces within my being to realign and submit, to let go of all the cogent fears and just exist ... and that innate decision saved me a lot of psychic damage. What is most outstanding about the way it feels is an inability to judge in any way, by any method of the mind ... it is unconquerable, as deep and profound as a totally unconditional love that is life. What a trip, huh?"
(with 15 mg, smoked) "At about 60 seconds after I smoked this free base, I beheld every thought that was going on everywhere in the universe and all possible realities while I was wracked out with this horrible ruthless love. It scared the hell out of me. When I could see again (15 minutes later) it was almost as if there was an echo of a thought in my head saying that I was given an extremely rare look at the true consciousness of it all. I've never been hit this hard since then. A definite ++++."
(with perhaps 20 mg, smoked) "This is a very strong hallucinogen. A twenty minute experience. For me it was like adding the MDMA experience to DMT. DMT for me is terrifying (I still go back though) and I must really think about it before proceeding. The 5-MeO-DMT was much more relaxed, a kind of cosmic consciousness type of experience. I broke into a space similar to DMT but it was more like receiving grace. I felt a little shaky (tremor-like) coming down."
(with 25 mg, smoked) "I placed 25 mg of 5-methoxy-DMT in a stainless steel one-quarter teaspoon and vaporized it over a cigarette lighter collecting the smoke in an upside-down funnel. All smoke was inhaled; the taste was mild -- none of the plastic taste of DMT. About 10 seconds or so after inhaling the last of the smoke, it began with a fast-rising sense of excitement and wonder, with an undertone of "Now you've done it," but dominated by a sense of, "WOW, This Is IT!" There was a tremendous sense of speed and acceleration In perhaps 10 more seconds these feelings built to an intensity I had never experienced before. The entire universe imploded through my consciousness. It's as if the mind is capable of experiencing a very large number of objects, situations and feelings, but normally perceives them only one at a time. I felt that my mind was perceiving them all at once. There was no distance, no possibility of examining the experience. This was simply the most intense experience possible; a singularity, a white-out (as opposed to a black out), I have little memory of the state itself. I have no memory, for example, of whether my eyes were opened or closed. After some seconds or minutes , it started to fade and came to resemble a merely intense psychedelic state. Here I had the feeling, a visualization of being part of the universe of beings, all active in our daily, interwoven tasks, still moving at an incredible rate, and with a longing for a single group / organism awareness and transcendence. In a few more minutes it faded to an alert (+ one) state with an additional sense of awe and wonder, relief, and a strong feeling of gratitude toward the universe in general, for the experience."
(with 30 mg, smoking) "I placed approx. 30 mg of 5-MeO into a pipe, and smoked it, in one toke, without a second thought. An instant later, I was crawled up on my bed (in the fetal position) with my eyes closed, squirming around, screaming (in my head) 'Fuck! You killed yourself!' I repeated this several times, very fearful of death. I didn't see anything, while my eyes were shut, except for a bright white light, that which you see after staring at a bright light. The only other "vision" was one in my mind - I came to the realization that my life would be wasted if I died there. This showed me all of my scripts being discarded and nothing good happening ever again. It was a glimpse into my future, if I died. I concentrated on breathing and that helped me survive (mentally). I walked into the living room and placed a CD into the stereo, and as the first song started, my attention span disappeared, and I walked back into my bedroom. To my surprise, forty minutes had passed, in what I remembered as mere seconds. This scared me, thinking that maybe I had blacked out. I felt the effect for about an hour, it slowly faded away."
(with an unknown but large amount, smoked) I observed the subject pass very quickly into an almost coma-like state. Within seconds his face became purple and his breathing stopped. I pounded his chest, and breathed for him, and he seemed to emerge in consciousness, with the comment, "This is absolute ecstasy." He stopped breathing a second time, and both heart massage and mouth-to-mouth resuscitation was provided. Again, he recovered and managed to maintain a continuing consciousness and achieve a partial recovery. In the awake condition he was increasingly lucid, but on closing his eyes he became possessed with, what he called, "The energy of terror." He could not sleep, as upon closing his eyes he felt threatened in a way he could not tolerate. Three days later, medical intervention with antipsychotic medication was provided, which allowed the recovery of an acceptable behavior pattern in a few more days.
(with 35 mg, orally): "No activity."
(with 0.25 mg, i.v.): "A real effect."
(with 0.5 mg, i.v.) "I felt the effects distinctly within a minute, along with some pain at the injection site. In a few minutes I felt a very distinct calming and stilling of my mind. While I could have carried on a conversation about anything and didn't feel the least bit stoned, I found the feeling very recognizable."
(with 0.7 mg, i.v.): "This was basically a +1 experience. After a few instances I felt its motion, very gentle waves. I was thinking about thinking about the experience, about writing about it, and so I was experiencing myself as both observer and editor. This was not overwhelming, but gentle."
(with 1.3 mg, i.v.) "In a few seconds I was participating in exquisite, full body, teeth-chattering shivers that lasted in all about 10 minutes, nearly the duration of the effects. The sensations seemed to come more from my head region, whereas my 'full blown' experiences of smoked 5-MeO-DMT seemed to emanate from my center and heart."
(with 2.3 mg, i.v.) "I remember having a perspective of knowing I was aware and, if not from the start of the experience then very soon into it, knowing I knew I was aware. I thought I was an ocean. I don't remember where I first lost continuity of consciousness (this is a little like a black-out from hard liquor) but I remember being aware of the sounds I was making apparently some time after I began vocalizing. Around the time I thought to change these sounds as I pleased, I also noted with brief wonder that the sound was continuous, not changed by my breathing. I sang my way back."
(with 3.1 mg, i.v.) "I vocalized effortlessly. I was getting in touch with my body. I said, 'Turn off the lights,' and 'I love you,' and then I lost it. I was amazed later to find a roomful of people who had been frightened by the noises they had been amazed to hear, and I was amazed to be told I had made."
EXTENSIONS AND COMMENTARY : This is, like DMT, another naturally occurring alkaloid that is not orally active. And, as with DMT, it is almost always smoked. This is the reason that both there and here, there are several entries with the word "perhaps" in the dosage statement. When the transportation vehicle is a rolled joint containing some inert plant carrier, or a glass pipe heated with a torch, who can accurately say how much of the drug was actually volatilized and drawn into the lungs? Further, from the qualitative range of responses, one can truly say, it is different things to different folks. I don't know of any active oral level (I have been told of it being tried at 35 milligrams) but a number of experiments with oral 5-MeO-DMT preceded by harmaline have shown activity in the 10 - 25 milligram area. These are discussed in the Hoasca vs. Ayahuasca chapter. Some trial i.v. experiments with radioactively labeled and unlabeled materials showed no effects at 100 micrograms, but real effects at 250 micrograms. Higher levels have convincingly established the enhanced potency that is the result of this route. The injection process is faster than the smoking process, and it avoids the smoke's odd flavor.
5-MeO-DMT was first observed in a member of the Rue family (Rutaceae) called Dictyoloma incanescens. Now it is recognized as a major component of a number of South American snuffs. The snuff called cohoba is generally associated with plants of the Piptadenia and Mimosa genus, and as they are largely DMT-containing, they are discussed under that entry. But there are other snuffs, such as yakee and yato (in Colombia) and paricá, epená and nyakwana in Brazil, which should probably be discussed here. The plants used are of the Virola genus, containing trees most plentifully found in the Amazon basin.
There has been a long-standing and never-to-be-resolved disagreement amongst botanists as to the best way of classifying plants. There are the morphotaxonomists, who insist that species assignment should be based primarily on appearance, and there are the chemotaxonomists who feel that the natural composition should be a deciding factor in the distinction between species. But the ultimate requirement for morphology is to find the plant in bloom, and for chemistry, to have some analytical capabilities. Often, neither luxury is available in the jungles of the rain forest. A major contributor to the Virola snuffs, Virola theiodora, is a good case in point. Two plant sources, both gathered in Brazil, have been found to have radically different compositions. In one, 5-MeO-DMT is substantially the only alkaloid found in the bark, whereas in the other, DMT is the major alkaloid. But both have DMT almost exclusively in the young green shoots. Are they the same species? Another plant used in some of the snuff preparations is Virola calophylla, where the bark, root, leaves and shoots all run about 90% DMT as the alkaloid content. Yet, the alkaloids in the root and bark of Virola rufuta consist of some 95% 5-MeO-DMT.
The inquiries into metabolic 6-hydroxylation as a prelude to biological activity have been made with both 5-MeO-DMT and the corresponding primary tryptamine (see below). 6-HO-5-MeO-DMT has been shown in several animal models to be pharmacologically less active than its parent compound. See the discussion under DET for the role that this metabolism played in some early clinical studies.
Removing one of the N-methyl groups provides N-methyl-5-methoxytryptamine (5-MeO-NMT), which has its own entry. Removal of both methyl groups from the nitrogen gives 5-methoxytryptamine (5-MeO-T) which has been explored most extensively by Soviet researchers as a treatment for exposure to radiation; this aspect of its action is discussed and expanded upon in the commentary under Melatonin. It is also known by the trade name Mexamine and has been looked at as a potentiator of centrally active drugs. Here, as with the simpler N,N-dialkyltryptamines, the metabolic introduction of a hydroxyl group at the 6-position (to give 6-HO-5-MeO-T) leads to a lowering of pharmacological potency. And again, no human studies have been reported.
A true academic challenge exists with the many studies of 5-methoxy-DMT (as has been mentioned under DMT, different drug, same problem) which have involved drug mixtures. The second drug, added to the tryptamine, is almost always a monoamineoxidase inhibitor such as harmaline, either as a chemical (in most clinical studies in the Northern Hemisphere) or as the plant decoction (in most jungle uses in the Southern Hemisphere). The challenge is, just how should one classify these observations? Under the first drug modifying or being modified by the second? Under the second drug modifying or being modified by the first? Or should the mixture be treated as a variable thing in its own right?
Since the mixture invariably shows properties that neither component can show alone, it is obvious that the combination is a major classification component. When the harmaline component is a plant mixture containing harmaline, a common name that is used is Ayahuasca. This can be any of a large number of carboline-containing plants (or even harmaline itself) combined with a really wide variety of amines ranging from the tryptamines to mushrooms to such diverse materials as Jimpson Weed components. These combinations are usually unknown as to exact composition, and they will be discussed in a chapter devoted to just this combination, entitled "Hoasca vs. Ayahuasca." On the other hand, when the components are discrete compounds, the process is much more controlled (in the experimental sense rather than in the effects sense) and these combinations are gathered in the recipe for harmaline.
There are a couple more entries for 5-MeO-DMT, one very important one, and the other quite trivial. There is a drug-use phenomenon that is often referred to by the popular title of "toad-licking." The toad involved is the Sonora Desert Toad, also called the Colorado River Toad, and carries the binomial Bufo alverius. It is not the closely related marine toad Bufo marinus, as some people have insisted, prompted by the early Olmec and Mayan iconography. Of course the licking myth is newspaper hype -- it is the venom that is active, and it is smoked. When the desert toad is stroked near the parotid glands in the neck region, there is the squirting out of this venom and when it is allowed to dry on a hard surface it takes on the texture of rubber cement. It contains up to 15% 5-MeO-DMT, as well as N-methyl-5-methoxytryptamine, 5-MeO-NMT and Bufotenine, which have their own entries.
And here is the trivial entry. I involved myself in a small Australia / toad incident when I recently visited Sydney. There is a consistent historical record of the axiom "the road to Hell is paved with good intentions" in the effort to import solutions to problems that were the unforeseen consequences of earlier imported solutions. I can't remember the decade-by-decade record but as I remember it involved, amongst other things, dogs, rabbits, viruses to control rabbits, and maybe mongooses. And cattle. Cattle had been imported in mid-century as a desired agricultural commodity, but it could not be predicted that their cow-plops would not deteriorate. There were domestic dung beetles, but they appreciated kangaroo droppings (raisin-sized) rather than cow-plops (birthday-cake sized). So the eggs of a cow-oriented dung beetle were brought in about 1970 and, after weathering the usual quarantine, were released into the ecosphere. Another beetle came in without invitation with the importation of agricultural cane. Hitch-hiking along with the cane was a cane beetle, and it had no natural enemies. The beetle proliferated, and as a solution to this infestation there was brought in a "cane toad," the Bufo marinus (the marine toad, not the desert toad, to the eventual disappointment of the drug-oriented subculture) which was believed could provide some control over them. Well, it turned out that the beetles lived at the top of the cane stalks, and the frogs lived at the bottom. The toad didn't eat the beetles, but they did successfully reproduce and multiply, because they, too, had no natural enemies. They are today sweeping across north-eastern Australia.
In the middle of downtown Sydney, right alongside Hyde Park at Williams and College, there is the Australian Museum, with a super library of natural history which I wished to use in the pursuit of the Aborigine use of red beans. And there was a special exhibit on display of the frogs and toads of Australia, with histories, photographs, and occasional sound tracks of croakings. I spotted a panel devoted to the origins and short history of the Bufo marinus. And right in front of it was a little old lady diligently reading the text which said, approximately, that a virus was being developed at some research laboratory in South America, that would be specific for this toad and which would bring the problem under control. I wondered to myself, but just loud enough for her to hear, if this was the same virus that could cause the AIDS syndrome in the Wallaby?
She looked at me for a moment, turned, and walked away. Maybe, just maybe, another rumor of unknown origin has been launched.